

The alkaline earth elements are metallic elements found in the second group of the periodic table. The alkali metals are softer than most other metals. As with all metals, the alkali metals are malleable, ductile, and are good conductors of heat and electricity. Therefore, they are ready to lose that one electron in ionic bonding with other elements. These metals have only one electron in their outer shell. The alkali metals, found in group 1 of the periodic table, are highly reactive metals that do not occur freely in nature. For our purposes we will define the following ten families: The Periodic table can also be divided into several families of elements each having similar properties. Elements of each group had similar properties. The modern one states that the properties vary with atomic number, not weight.Įlements in Mendeleev's table were arranged in rows called periods. His periodic law states that the chemical and physical properties of the elements vary in a periodic way with their atomic weights. He observed that many elements had similar properties, and that they occur periodically. Mendeleev created the first periodic table based on atomic weight. They both created similar periodic tables only a few months apart in 1869.
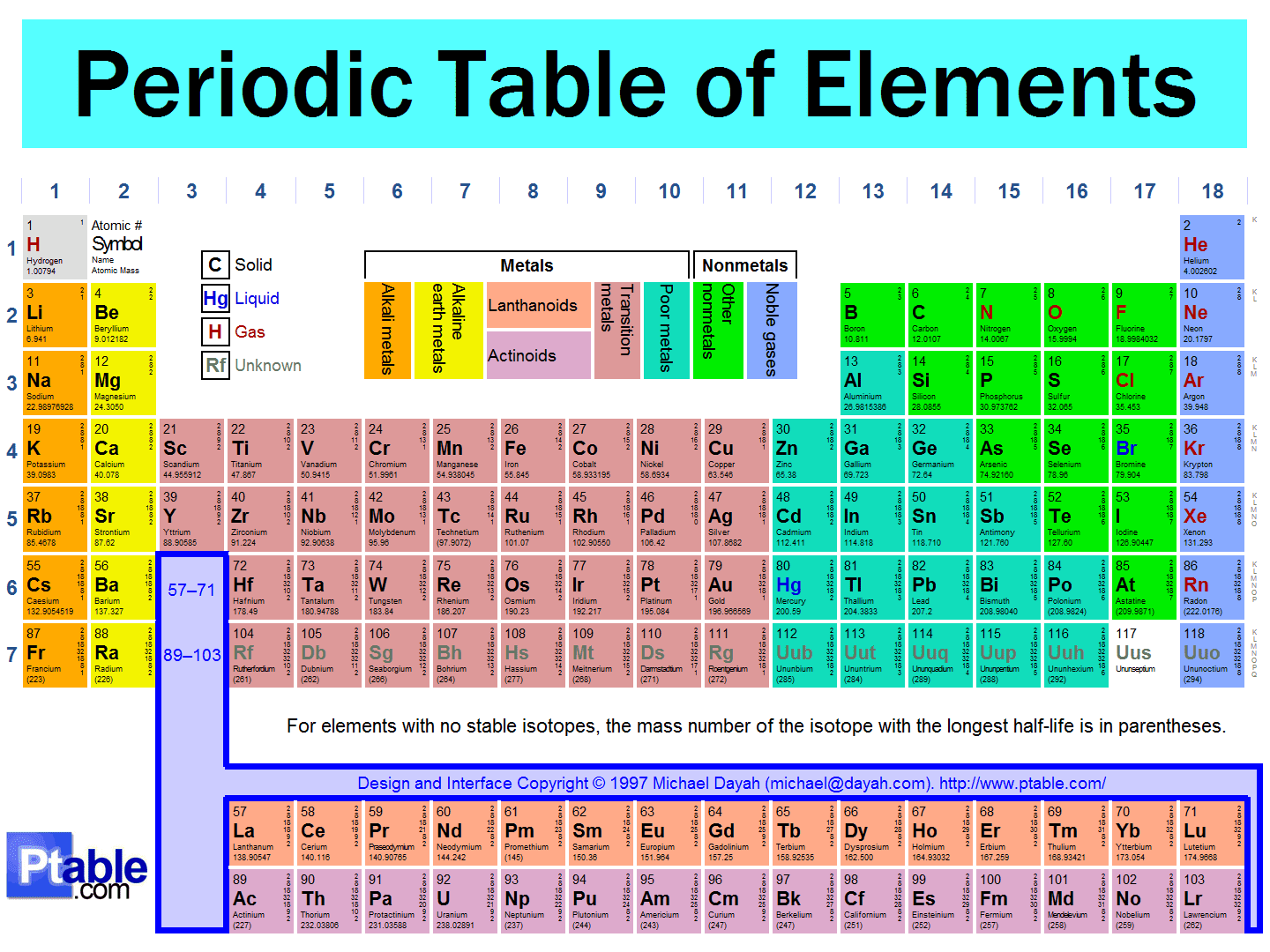
The modern periodic table, based on atomic number and electron configuration, was created primarily by a Russian chemist, Dmitri Ivanovich Mendeleev, and a German physicist, Julius Lothar Meyer, both working independently.

Chemistry and Periodic Table Applications


 0 kommentar(er)
0 kommentar(er)
